AmpleLogic RIMS
Regulatory Information Management System
Empower Your Regulatory Journey with AmpleLogic RIMS
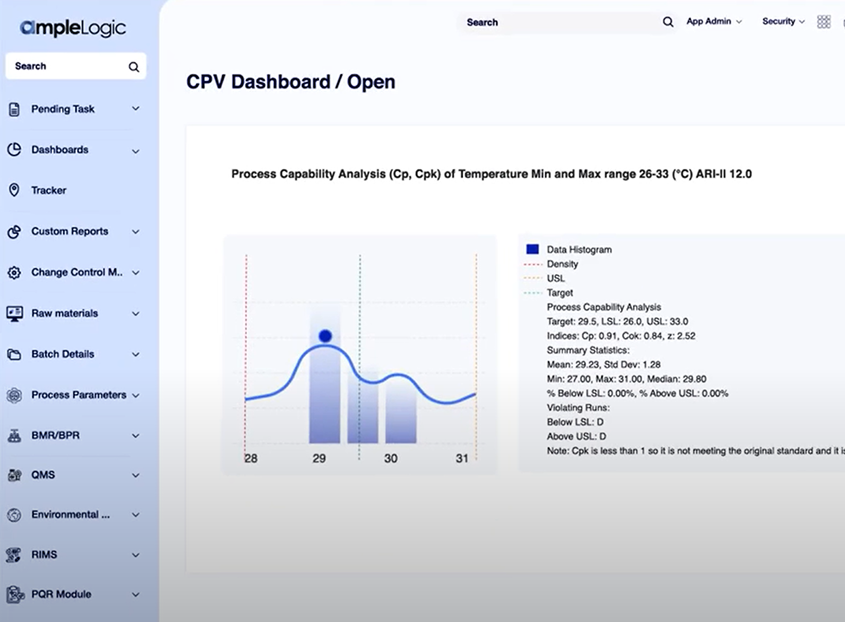
AI-Powered APQR Software
Annual Product Quality Reviews, Reimagined with AI
Annual Product Quality Review is a critical regulatory requirement for pharmaceutical and life sciences organizations. AmpleLogic’s APQR solution enables structured, compliant, and inspection-ready reviews by consolidating quality data, automating trend analysis, and generating audit-ready reports.
Trusted by 100+ Pharma Companies Globally






















Why Choose AmpleLogic RIMS?
AmpleLogic Regulatory Information Management System distinguishes itself as a premier system optimising regulatory activities from product inception to market launch. With features like regulatory submission tracking and validation, change control management, graphical report generation, our software eliminates manual work and ensures adherence to worldwide regulatory standards like 21 CFR Part 11 and EU Annex 11.

Streamlines operations
Simplifies regulatory compliance and enhances efficiency

Integration capabilities
Seamless integration for data exchange and data consistency

Compliance Assurance
Minimize compliance risks and reduce wastage

Electronic Authentication
Accomplish regulatory mandates with e-signatures and audit trails
Optimize Your Regulatory Workflow with AmpleLogic RIMS
Pharmaceutical companies face challenges in managing regulatory information due to evolving standards, fragmented data, and the risk of compliance failures, which can lead to penalties, delays, or recalls. Many Regulatory Information Management Systems (RIMS) still rely on manual processes, increasing the chances of errors and delays. As companies grow, scaling RIMS to manage increasing data volumes and maintain audit readiness becomes crucial.

Efficient Lifecycle Management
Navigate the complexities of Regulatory Information Management effortlessly.

Redundancy Reduction
Wave off manual efforts and experience streamlined efficiency

User-Friendly Interface
Experience intuitive and user-friendly interface for simplified report submissions

Dynamic Compliance
Adapt seamlessly to the evolving regulatory landscape
AmpleLogic’s AI-powered RIMS solution transforms this process by automating compliance tracking, ensuring alignment with changing standards, and centralizing data for easy access and updates. The AI uses predictive analytics to identify and mitigate risks, automates manual tasks, and reduces errors, accelerating reporting and meeting deadlines. Scalable and efficient, the solution adapts to business growth, ensuring audit readiness and minimizing operational costs, helping companies stay compliant while driving innovation.

Regulatory Submission Tracking
Monitor submission progress with automated tracking features, reducing manual follow-ups

Commitment & Variation Management
Effectively manage commitments, product variations, and regulatory obligations with tracking tools
Challenges Tracking Pharmaceutical Regulatory Information

Complex and Evolving Regulations
The pharmaceutical industry is governed by complex and frequently changing regulations, both at local and global levels. Keeping up with these changes is time-consuming and difficult
Scalability
As companies grow, regulatory data volume and complexity increase, demanding scalable systems that adapt to evolving requirements and support expanding business needs.

Compliance Risks
Non-compliance can result in significant penalties, delays, and reputational damage. Ensuring that all regulatory requirements are met across different regions is a constant challenge

Manual Processes
Many organizations still rely on manual processes for tracking and managing regulatory data, which increases the risk of human error, delays, and missed updates

Data Security and Privacy
Regulatory data must be protected from unauthorized access and breaches, which can be a challenge when dealing with large volumes of sensitive information

Audit Readiness
Keeping regulatory documentation organized, up-to-date, and ready for audits requires significant effort and resources, especially during inspections
Case Studies
Effortless Compliance, Elevated Efficiency

Compliance Tracking
AI continuously monitors and adapts to evolving local and global regulatory standards, automatically updating processes to ensure compliance without manual intervention.

Predictive Analytics
Leveraging AI, the solution analyzes historical and real-time data to identify potential risks and compliance gaps before they escalate.

Reporting & Documentation
Automates generation of regulatory reports and documentation, ensuring accuracy, timeliness, and audit readiness with minimal human involvement.

Risk Mitigation
AI-powered algorithms assess data patterns to flag potential compliance risks and recommend corrective actions before issues arise.

Regulatory Change Management
Tracks regulatory changes in real time and automatically implements them to ensure ongoing compliance with the latest standards.

Enhanced Efficiency
Optimizes workflows, reduces operational costs, accelerates regulatory processes, and ensures consistent compliance across the organization.

Tracking and Publishing of Submissions
Monitor, track, and publish regulatory submissions efficiently within the system.

Reviewing Submissions
Dedicated functionality for systematic review of submissions before finalization to ensure compliance.

Approval and Rejection Monitoring
Real-time tracking of approval or rejection status for each submission to streamline decision-making.

FDA Target Date Notifications
Notifications for FDA target dates to ensure timely compliance with critical regulatory deadlines.

Product Registration
Collect and manage product registration information seamlessly within the system.

Superb Authentication
Validate records using e-signatures, timestamps, and role-based access for enhanced security.

Submission Validation
Ensures compliance and technical accuracy of documents prior to regulatory submissions.

Automatic Notifications for Critical Dates
Receive timely alerts for FDA target dates and other compliance milestones.

Cost Reduction
Reduce operational expenses while improving productivity and efficiency.

Submission Preparation & Management
Create, track, review, and publish submissions easily with real-time approval monitoring.

Change Control Management
Tracks and regulates changes to regulatory documents, ensuring full traceability throughout the lifecycle.

Graphical Reports
Interactive graphical reports for easier analysis and informed decision-making.

Real-time Notifications
Receive instant email notifications for regulatory commitments and updates.

Product Registration Tracking
Easily collect and update product registration data for accurate regulatory reporting.

Centralized Database
Access a centralized database from anywhere for streamlined information retrieval.

Vendor Management
Manage and coordinate third-party vendors to ensure regulatory compliance.

Product Lifecycle Management
Manages product development lifecycle from regulatory approval to market release.

Cross-Industry Compatibility
Applicable across industries including life sciences, food & beverages, and cosmetics.

Workflow Automation
Automates repetitive regulatory processes to reduce turnaround time and compliance risks.

Audit Trials
Maintains audit trails of system modifications to support accountability and forensic audits.
Switch to AmpleLogic AI RIMS Solution
AmpleLogic AI-powered RIMS automates regulatory tasks, ensures compliance, accelerates approvals, and provides audit-ready reports. Compliant with 21 CFR Part 11 and EU Annex 11, it enhances efficiency, reduces costs, and optimizes the regulatory process from start to launch.
Industries We Serve
Industry-Specific Software for Enhanced Quality and Compliance

Life Sciences
Benefit from GMP solutions customized for life sciences, Pharma, R&D, biotech etc.

Pharmaceuticals
Enhance cannabis and tobacco manufacturing with advanced software for efficiency.

Medical Devices
Transform the medical device industry with software that enhances compliance.

Gene & Cell Therapy
Transform gene therapy with advanced software for precision and efficiency.

Food & Beverages
Enhance food and beverage manufacturing with software for efficiency and operational agility.

Cosmetics
Boost cosmetics and beauty operations with innovative software for process optimization.
Case Studies
Why Choose AmpleLogic RIMS?
AmpleLogic Regulatory Information Management System distinguishes itself as a premier system optimising regulatory activities from product inception to market launch. With features like regulatory submission tracking and validation, change control management, graphical report generation, our software eliminates manual work and ensures adherence to worldwide regulatory standards like 21 CFR Part 11 and EU Annex 11.

Streamlines operations
Simplifies regulatory compliance and enhances efficiency

Integration capabilities
Seamless integration for data exchange and data consistency

Compliance Assurance
Minimize compliance risks and reduce wastage

Electronic Authentication
Accomplish regulatory mandates with e-signatures and audit trails
Our compliance
Why Choose us?
Our compliance-first, AI-enabled platform is designed to meet the rigorous demands of regulated industries, enabling faster application.

10X Faster Deployment
Our platform accelerates solutions, reaching markets 10x faster.

ROI in 3 Months
Low-code solutions deliver fast deployment, cost savings, & quick ROI.

98% Project Rate
Experience a 98% success rate, surpassing the industry average of 56%

Domain Expertise
We deliver tailored solutions, ensuring business growth and lasting success
FAQ’s
Get Answers to All Your Queries
RIMS is a Regulatory Information Management System designed to streamline and simplify the product application and registration lifecycle in various industries.
AmpleLogic RIMS reduces manual efforts, eliminates redundancy, and offers a user-friendly interface, resulting in increased efficiency in regulatory processes.
AmpleLogic RIMS adheres to electronic record requirements established by regulatory authorities worldwide, including TGA, CDSCO, HEALTH CANADA, MCC, ANVISA, EMEA, SFDA, NAFDAC, MEDSAFE, MHLW, MCAZ, SWISSMEDIC, and KFDA.
AmpleLogic RIMS caters to industries such as pharmaceuticals, biotechnology, medical devices, food & beverages, and more.
Yes, AmpleLogic RIMS is scalable and adaptable, catering to the needs of businesses of all sizes – from small to large enterprises.





