AmpleLogic eLogbook
Electronic Logbook System
Digitize Your Operations with AmpleLogic AI Powered eLogbook
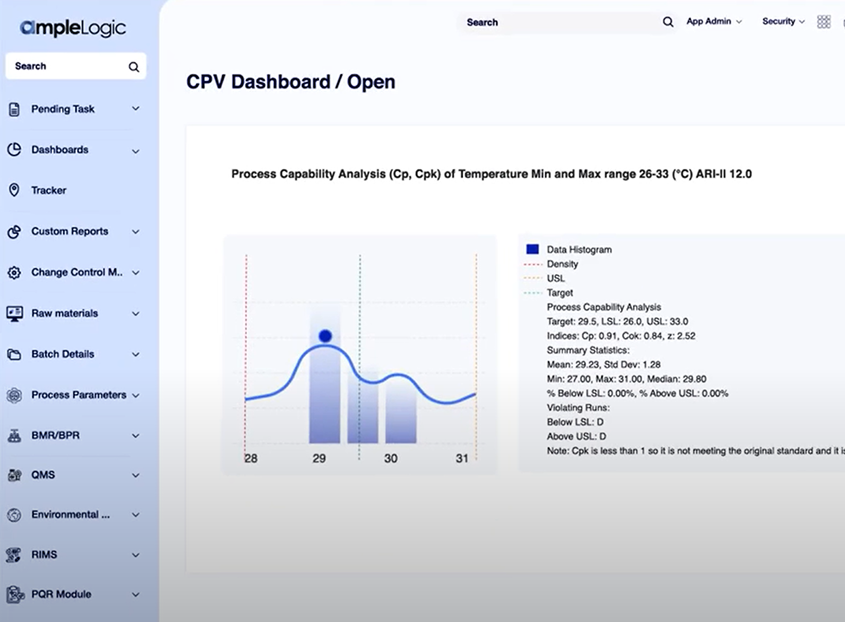
AI-Powered APQR Software
Annual Product Quality Reviews, Reimagined with AI
Annual Product Quality Review is a critical regulatory requirement for pharmaceutical and life sciences organizations. AmpleLogic’s APQR solution enables structured, compliant, and inspection-ready reviews by consolidating quality data, automating trend analysis, and generating audit-ready reports.
Trusted by 100+ Pharma Companies Globally






















Dive into the power of AI Intelligent Pharma Digital Logbook Software!
The Lifesciences sector, including pharmaceutical, biotech, and related industries, face significant challenges in managing operational logs. Despite availability of advanced digital solutions, there is a lack of intelligent systems that accommodates the needs of the regulated sectors and ensures operational efficiency. These outdated systems amplify compliance risks and hinders efficiency in an industry where overall performance, precision and adherence to standards is paramount.
AmpleLogic’s AI-enhanced eLogbook Solution revolutionizes operational logging for Lifesciences, Food & Beverages, Cosmetics, Gene Therapy, and Medical Devices industries. With AI-driven anomaly detection, seamless log entries via voice or text, and real-time regulatory insights, the solution proactively mitigates risks and ensures compliance with global standards like FDA 21 CFR Part 11, EU Annex 11, MHRA, GAMP, GMP, and ISO. Improve operational efficiency and audit readiness, all while maintaining the highest standards of compliance.
Why choose AmpleLogic AI Pharmaceutical eLog book?
AmpleLogic’s AI-powered eLogbook software is a game-changer in the pharmaceutical and life sciences industries, reimagining how logbook management is handled. With the power of artificial intelligence and automation, it offers a seamless and efficient solution that simplifies data logging, ensures stringent regulatory compliance, and streamlines operations. Imagine a system where voice commands replace tedious manual entries, making real-time data logging effortless and accurate. The software’s anomaly detection feature automatically flags discrepancies, ensuring data integrity while maintaining compliance with industry standards. It doesn’t stop there—predictive maintenance anticipates equipment failures before they happen, minimizing downtime and safeguarding productivity.
With customizable templates, organizations can tailor their logbook entries to suit specific needs, while advanced data insights help unlock hidden opportunities to optimize processes. Fully compliant with FDA, GMP, and WHO guidelines, AmpleLogic’s Electronic Logbook Software not only reduces errors but also enhances data accuracy and boosts operational efficiency, offering a smooth transition from paper-based logbooks to a smarter, digital approach.
Comes with all new AI features!
Voice Control
Simplifies data logging and retrieval with voice commands or natural queries

Intelligent Data Insights
Analyze trends to uncover inefficiencies and optimize operations

Anomaly Detection
Spots data outliers to identify errors or compliance issues
Predictive Maintenance
AI predicts equipment failures with alerts to prevent downtime and ensure compliance

Streamline Data Management
with AmpleLogic e Logbook

Digital Record Keeping
Replaces manual logs with secure digital records, eliminating errors, reducing storage needs, and simplifying audits.

Voice-Enabled Logging
Effortlessly log data using voice commands, making real-time data entry faster and more accurate.

Equipment & Area Data Management
Tracks equipment usage, environmental conditions, and maintenance activities to ensure compliance and operational efficiency.

Personalized Recommendations
AI-driven insights based on usage patterns to optimize workflows, scheduling tasks, and resource utilization.

Real-Time Monitoring
Real-time tracking of temperature, humidity, and equipment performance to ensure continuous compliance.

Customizable Workflows
Tailors logbook entries and approval workflows to meet specific operational requirements with improved accuracy.

Competency Records
Maintains employee training and competency records to ensure a skilled and compliant workforce.

Data Security
Ensures robust security with encryption and role-based access controls to protect sensitive data.

Audit Trail
Maintains an immutable audit trail of all actions, approvals, and changes for regulatory compliance.

User-Friendly Interface
Intuitive interface designed to reduce learning curves and improve user adoption.

Smart Report Generation
Generates custom reports in PDF, CSV, or XML formats with automated scheduling for timely updates.

Integration Capabilities
Seamlessly integrates with ERP, MES, LIMS, and other enterprise systems for smooth data flow.

Regulatory Compliance
Complies with FDA 21 CFR Part 11, EU Annex 11, and GMP standards to maintain data integrity.

Automated Alerts & Notifications
Sends automated reminders for calibration, maintenance, and compliance activities.

Maximum Traceability
Tracks operations end-to-end, ensuring full visibility and accountability.

Area Monitoring and Control
Restricts unauthorized access to sensitive manufacturing areas using controlled permissions.

Cleaning and Alarms
Triggers cleaning schedules and alarms based on usage and regulatory requirements.

Roles and Departments
Configures role-based access and permissions across departments for better governance.

Version Control
Maintains complete document version history with a clear audit trail.

Enhanced Analytics
Visual dashboards and analytics to monitor trends and performance metrics.

QR & Barcode Scanning
Enables real-time tracking using QR and barcode scanning.

Verification
Ensures equipment, rooms, and solutions are verified and approved before use.

Document Management
Centralized document templates for SOPs and compliance documentation.

Calendar View for Logs
Displays log entries in a calendar format for easy scheduling and tracking.

Interlocking
Safety interlocks prevent unauthorized or unsafe equipment operations.

Access from Any Device
Access the eLogbook from any internet-enabled device for flexibility.

User Tracking
Tracks user activity to improve usability and identify training needs.

Offline Access
Offline data entry with automatic sync when connectivity is restored.

Logbook Entry Upload/Download
Upload and download log entries using authorized formats and templates.

Exception Handling
Detects, records, and resolves irregularities accurately.

Authentication & Traceability
Active Directory integration, biometrics, and barcode authentication ensure enhanced security and traceability.
Switch to AmpleLogic’s AI-Powered eLogbook Solution
AmpleLogic’s AI-powered eLogbook transforms pharmaceutical logbook management with real-time voice logging, anomaly detection, predictive maintenance, and customizable templates. Fully compliant with FDA, GMP, and WHO guidelines, it ensures accuracy, efficiency, and a seamless shift to digital operations.
Industries We Serve
Industry-Specific Software for Enhanced Quality and Compliance

Life Sciences
Apply Nelson Rules to detect abnormal process behaviour and statistically significant trends.
Pharmaceuticals
Ensure consistent and inspection-ready APQR execution aligned with regulatory requirements.

Medical Devices
Manage APQRs with controlled documentation, version history, and traceability to support quality system.

Gene & Cell Therapy
Address the complexity of advanced therapies by capturing batch-specific data, deviations.

Food & Beverages
Conduct structured product quality reviews that support safety, consistency, and regulatory expectations.

Cosmetics
Maintain standardized APQR documentation and quality trend visibility to support regulatory.
Case Studies
Our compliance
Why Choose us?
Our compliance-first, AI-enabled platform is designed to meet the rigorous demands of regulated industries, enabling faster application.

10X Faster Deployment
Our platform accelerates solutions, reaching markets 10x faster.

ROI in 3 Months
Low-code solutions deliver fast deployment, cost savings, & quick ROI.

98% Project Rate
Experience a 98% success rate, surpassing the industry average of 56%

Domain Expertise
We deliver tailored solutions, ensuring business growth and lasting success
FAQ’s
Get Answers to All Your Queries
AmpleLogic offers a comprehensive digital logbook software solution, replacing manual paperwork with automated processes, ensuring accuracy and compliance.
Yes, our eLogbook is fully compliant with FDA regulations, including 21 CFR Part 11, ensuring the highest standards in electronic record-keeping.
By automating tasks, providing real-time visibility, and streamlining workflows, eLogbook significantly reduces manual efforts and enhances overall efficiency.
Absolutely. Our eLogbook is designed with flexibility in mind, allowing for customization to meet the unique requirements of different industries and organizations.
Security is a top priority. eLogbook employs robust measures to ensure data security and integrity, preventing unauthorized access and maintaining the accuracy of logbook entries.





