AmpleLogic CVS
Best Cleaning Validation Software in Pharma | AmpleLogic
Simplify Cleaning Validation with AmpleLogic AI-Driven CVS
AI-Powered APQR Software
Annual Product Quality Reviews, Reimagined with AI
Annual Product Quality Review is a critical regulatory requirement for pharmaceutical and life sciences organizations. AmpleLogic’s APQR solution enables structured, compliant, and inspection-ready reviews by consolidating quality data, automating trend analysis, and generating audit-ready reports.
Trusted by 100+ Pharma Companies Globally






















Enhance Cleanliness in Lifesciences powered by AI
Cleaning validation in the pharmaceutical and biopharma industries presents several challenges, from ensuring compliance with stringent regulatory standards to preventing cross-contamination between batches. Traditional methods often involve time-consuming processes, resource-intensive testing, and complex documentation. The need for effective residue detection, equipment cleanliness, and maintaining a high level of product quality adds to the complexity of cleaning validation.
AmpleLogic’s AI-powered Cleaning Validation Software addresses these challenges by offering a robust solution that streamlines the entire cleaning validation process. Designed specifically for the life sciences, pharmaceutical, and biopharma industries, it evaluates cleaning processes to ensure consistent product quality and compliance. The software leverages predictive analysis to detect potential issues early, optimize cleaning processes, and maximize resource efficiency. With real-time alerts for deviation identification, it enables swift corrective actions, ensuring compliance and maintaining operational efficiency.
Process Optimization
AI recommends efficient cleaning methods, saving time and resources

Automated Documentation
AI-generated accurate, compliant reports for audits

Resource Efficiency
Optimizes resource use, reducing waste and operational costs
Predictive Analytics
AI analyzes historical data to foresee potential issues and prevent cross-contamination

Features of AmpleLogic Cleaning Validation

Process Optimization
AI-optimized cleaning processes, saving time and resources while maintaining required cleanliness levels.

Data-Driven Decisions
AI analyzes extensive data to fine-tune cleaning protocols, driving enhanced consistency and continuous improvement.

Resource Efficiency
AI optimizes resource utilization, minimizing waste and reducing operational costs while maintaining effective cleaning outcomes.

Real-Time Alerts & Warnings
Instant alerts notify users of deviations, allowing for rapid corrective actions to prevent disruption in cleaning validation.

Protocol Creation & Management
Adaptive protocol creation and management for seamless execution, documentation, and tracking of validation activities.

Ensures Superior Product Quality
Facilitates regulatory compliance and quality assurance in pharmaceutical and other regulated industries.

Trend Analysis
AI identifies trends, patterns, and anomalies, predicting risks and uncovering areas for improvement in cleaning processes.

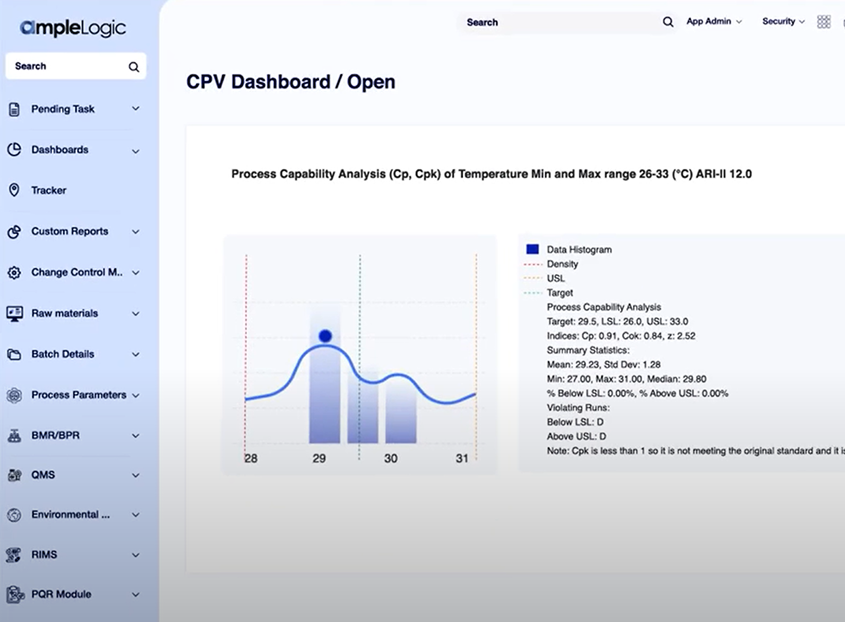
Continued Process Validation (CPV)
Machine learning models refine cleaning validation over time, reducing human error and driving continuous process improvement.

Risk Assessment & Mitigation
Identifies risks and implements mitigation strategies to ensure compliance with regulatory standards and safeguard product quality.

Maximum Allowable Carry Over (MACO)
Calculates MACO to determine acceptable residue levels after cleaning, minimizing contamination risks.

Seamless Integration
APIs enable smooth communication with other software systems, ensuring accurate real-time data exchange.

Effective Knowledge Transfer
Centralizes critical information and historical data, enabling continuous improvement and regulatory compliance.

Regulatory Compliance
Ensures full compliance with global regulatory standards including US FDA, EMA, TGA, and others.
Switch to AmpleLogic AI Pharma Cleaning Validation Software
Transform your pharma cleaning validation with AI-powered Cleaning Validation software. Real-time tracking, MACO-based contamination control, CPV, and predictive analytics ensure compliance with FDA, EMA, and cGMP standards. Automate residue detection, receive instant alerts, and optimize cleanliness, efficiency, and cost savings.
Key Challenges in Cleaning Validation in Pharma

Regulatory Compliance
Adhering to strict global regulations (FDA, EMA, cGMP) can be complex, requiring extensive documentation and regular audits
Cross-Contamination Risks
Ensuring there is no carryover of active pharmaceutical ingredients (APIs) or residues between batches, especially in multiproduct facilities

Residue Detection
Accurately detecting trace amounts of residue on equipment, especially for potent or low-dose drugs, presents a significant challenge

Variable Cleaning Effectiveness
The cleaning process can be inconsistent, leading to uncertainty in meeting required cleanliness levels across different equipment and batches

Lack of Standardization
Inconsistent cleaning protocols across different sites or production lines can lead to variability in results and compliance issues

Complex Equipment Designs
Cleaning intricate and hard-to-reach areas in complex manufacturing equipment can be time-consuming and resource-intensive
Case Studies
Why choose AmpleLogic Cleaning Validation Software?
Optimize Cleaning Validation Process with AmpleLogic’s brand new product! It comes with superior features that keeps track of cleaning processes and uses Maximum Allowable Carryover (MACO) to minimize risks of contamination. Continued Process Validation (CPV) ensures proper authorization and process enhancement over time.

Predictive Analysis
Predictive of past cleaning data help organizations anticipate issues, improve processes, and allocate resources better.

Excellent Interoperability
Communicate with other softwares and share data in real-time facilitating interoperability
Our compliance
Why Choose us?
Our compliance-first, AI-enabled platform is designed to meet the rigorous demands of regulated industries, enabling faster application.

10X Faster Deployment
Our platform accelerates solutions, reaching markets 10x faster.

ROI in 3 Months
Low-code solutions deliver fast deployment, cost savings, & quick ROI.

98% Project Rate
Experience a 98% success rate, surpassing the industry average of 56%

Domain Expertise
We deliver tailored solutions, ensuring business growth and lasting success
Challenges with Traditional APQR
Fragmented Data Across Multiple Systems

Manual Consolidation & Spreadsheet Dependency

Inconsistent Formats & Review Practices

Limited Visibility into Quality Trends

Increased Inspection & Compliance
FAQ’s
Get Answers to All Your Queries
GAMP stands for Good Automated Manufacturing Practice. It’s a set of guidelines and best practices for the pharmaceutical industry to ensure that automated systems are properly designed, validated, and maintained.
AmpleLogic GAMP Solutions provide compliant digital solutions that help organizations meet regulatory requirements through validated systems and documentation.
Compliance is ensured through validation frameworks, audit trails, documentation, and adherence to global regulatory guidelines.
aPaaS (Application Platform as a Service) enables rapid application development, deployment, and management in a compliant environment.
Key features include compliance-ready workflows, audit trails, validation support, scalability, and enterprise security.
Data integrity is ensured through access controls, encryption, audit logs, and compliance with ALCOA+ principles.





