AmpleLogic CAPS
AI-Powered Calibration & Preventive Maintenance Software
Streamline your processes with AI Intelligence
AI-Powered APQR Software
Annual Product Quality Reviews, Reimagined with AI
Annual Product Quality Review is a critical regulatory requirement for pharmaceutical and life sciences organizations. AmpleLogic’s APQR solution enables structured, compliant, and inspection-ready reviews by consolidating quality data, automating trend analysis, and generating audit-ready reports.
Trusted by 100+ Pharma Companies Globally






















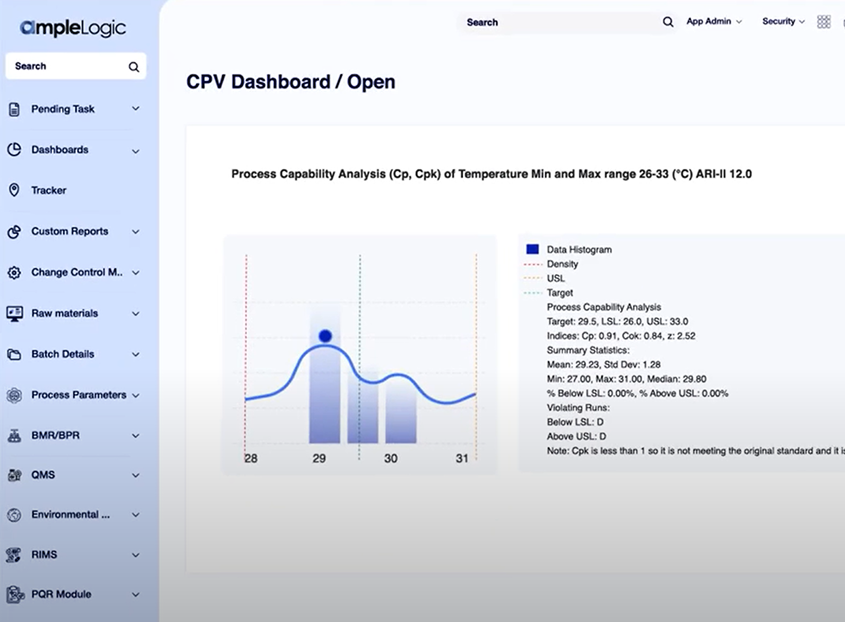
Enhance Calibration in Lifesciences with AmpleLogic AI CAPS Solution
The lifesciences and pharmaceutical industry faces challenges maintaining their instruments and equipment. Although various solutions exist in the market that helps in proper planning and execution of calibration and preventive maintenance, organizations need smarter solutions that need lesser input from the user and help in taking faster decisions.
Presenting AmpleLogic AI CAPS Solution – a unique application that provides you accurate suggestions for maintaining key resources. Significantly reduce time for planning maintenance processes for equipment failures and ensuring resources are in good condition. Manufacture the right quality products and comply with global regulations like 21 CFR Part 11, EU Annex 11, etc with AmpleLogic AI CAPS solution.
Commitment to AI-Powered Excellence
AI-based Maintenance Process Suggestion
AI suggests maintenance process for equipment/ instrument failure

Process Optimization
Efficient calibration and preventive maintenance of equipment

Calibration Forecasting
Displays upcoming deadlines for calibration or preventive maintenance
Reduced Time & Expenditure
Reduces 70% Time for approvals and ensures 100% cost reduction

Features of AmpleLogic AI CAPS

Annual Preventive Maintenance Schedule
Create annual Preventive Maintenance schedules for all equipment in production blocks and Quality Control departments, including large machinery and PLCs

AI-Powered Maintenance Insights
AI-Driven Maintenance Suggestions for Equipment Using Historical Data, Powered by BERT & LLaMA 3 Technologies

Eliminate Redundancies
No need to outline maintenance processes for similar equipment breakdowns every time. Simply choose from AI-driven suggestions powered by historical data for efficient resolutions

Visibility & Real-time Information
Renders complete visibility for usage logs and real-time information on the state of equipment and devices

Streamlined Equipment Disposal
Offers distinct process flow when an equipment is out of calibration or has been dumped. Provides procedure on discarding equipment as well

Record Maintenance
Maintenance records, calibration dates, and activities are meticulously logged, with the system retrieving prior calibration and preventative maintenance details

Alerts & Notifications
Get notified about upcoming preventive maintenance of equipment’s

Colour-Coded Calendar
Facilitates easy identification of calibrations and maintenance activities, displaying the current status, next due date and last calibration date

Enhanced Visibility
Examine ongoing and forthcoming calibration and preventive maintenance routines depending on location and department

Track Progress
Effectively track progress of inquiries for Out Of Calibration (OOC) records and take appropriate remedial action in a timely way

Calibration Date Generation
After successful calibration, generate calibration tags and certificates automatically

Report Generation
Automatically generate calibration reports

Detect Calibration Errors
Learn about calibration errors that occurred during the reporting period

Advance Integration
Learn about calibration errors that occurred during the reporting period

100% Enhanced Efficiency
Optimize processes with AI process recommendations for faster decision making in maintenance of pharma equipment/instrument

Cost Savings
Achieve 10x cost savings and 70% faster decision making with effective AI recommendations

Follow Regulations and SOPs
Keep records in accordance with authorized regulations and the SOPs of the organization
Built-In Insights Without External Tools
AmpleLogic APQR includes integrated analytics and dashboards to visualize quality trends, risks, and performance indicators eliminating dependency on external reporting tools.
Challenges in Pharma Equipment / Instrument Calibration & Preventive Maintenance

Regulatory Compliance
Adhering to stringent regulations set by FDA, EMA, and MHRA is challenging. Maintaining accurate documentation for audits and inspections is crucial to ensure compliance, operational efficiency, and regulatory readiness
Redundant & Slow Processes
For similar equipment/instrument failure, analysts need to outline maintenance processes every time and engage in multiple-level approvals. This lags decision-making, hinders operations and leads to big losses

Complexity of Equipment
Managing complex instruments needing specialized calibration, alongside maintaining calibration standards and reference materials, is crucial for ensuring accuracy and overcoming operational challenges

Time and Cost Constraints
Frequent calibration and maintenance must be balanced with operational efficiency. High costs, particularly for outsourcing calibration tasks, further strain resources

Scalability
Managing calibration tasks across multiple facilities is challenging. As organizations grow, scaling systems to meet increased demands becomes increasingly difficult

Audit Readiness
Ensuring calibration and maintenance records are always audit-ready is a significant challenge. Retrieving historical data quickly during inspections can be time-consuming
Case Studies
Why choose AmpleLogic AI CAPS Software?
Optimize pharma equipment/ instrument calibration and preventive maintenance with AmpleLogic AI CAPS! With AI recommended processes ensure faster maintenance of resources with significant cost savings. Additionally undertake calibration scheduling and forecasting, get real-time alerts, streamline equipment disposal, etc. With AmpleLogic AI CAPS solution enhance operational efficiency, regulatory compliance and quality assurance!

Eliminate Redundancies
Skip repetitive maintenance planning—choose AI-driven suggestions based on historical data

Flexible Scheduling
Allows creation, updating, or rejection of schedules at any equipment life stage

Priority Alerts
Highlights critical preventive maintenance schedules for immediate attention

Regulatory Compliance
Complies with regulatory standards put forward by organizations such as FDA, PDA, EMA, etc
Our compliance
Why Choose us?
Our compliance-first, AI-enabled platform is designed to meet the rigorous demands of regulated industries, enabling faster application.

10X Faster Deployment
Our platform accelerates solutions, reaching markets 10x faster.

ROI in 3 Months
Low-code solutions deliver fast deployment, cost savings, & quick ROI.

98% Project Rate
Experience a 98% success rate, surpassing the industry average of 56%

Domain Expertise
We deliver tailored solutions, ensuring business growth and lasting success
FAQ’s
Get Answers to All Your Queries
GAMP stands for Good Automated Manufacturing Practice. It’s a set of guidelines and best practices for the pharmaceutical industry to ensure that automated systems are properly designed, validated, and maintained.
AmpleLogic GAMP Solutions provide compliant digital solutions that help organizations meet regulatory requirements through validated systems and documentation.
Compliance is ensured through validation frameworks, audit trails, documentation, and adherence to global regulatory guidelines.
aPaaS (Application Platform as a Service) enables rapid application development, deployment, and management in a compliant environment.
Key features include compliance-ready workflows, audit trails, validation support, scalability, and enterprise security.
Data integrity is ensured through access controls, encryption, audit logs, and compliance with ALCOA+ principles.





