AmpleLogic RSMS
Regulatory Surveillance Management System
Streamline Regulatory Surveillance with AmpleLogic RSMS
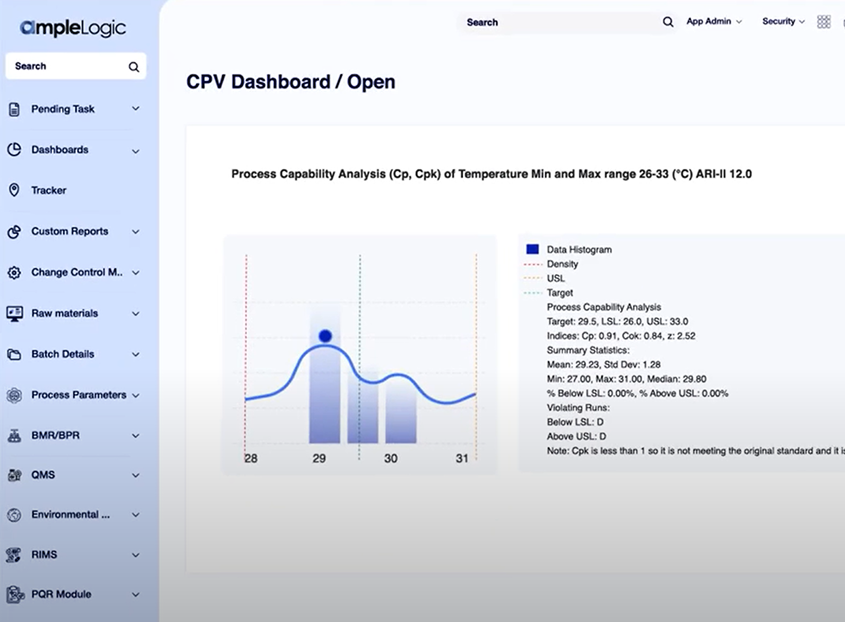
AI-Powered APQR Software
Annual Product Quality Reviews, Reimagined with AI
Annual Product Quality Review is a critical regulatory requirement for pharmaceutical and life sciences organizations. AmpleLogic’s APQR solution enables structured, compliant, and inspection-ready reviews by consolidating quality data, automating trend analysis, and generating audit-ready reports.
Trusted by 100+ Pharma Companies Globally






















Stay Ahead of Compliance with AmpleLogic’s RSMS System
In the rapidly evolving pharmaceutical industry, staying compliant with global regulations is a constant challenge. AmpleLogic’s Regulatory Surveillance Management System (RSMS) is a robust solution designed to monitor, assess, and manage regulatory changes proactively, ensuring seamless compliance with global authorities like FDA, EMA, MHRA, and other regulatory bodies.
GMP Trends
Monitor Good Manufacturing Practices (GMP) trends to align manufacturing processes with the latest industry standards.

No Objection Certificates (NOC)
Automate the tracking and management of NOCs required for product approvals.

Notice of Concern (NOC)
Identify and address potential compliance risks before they result in enforcement actions.
Cross-Border Compliance Management
Ensure seamless regulatory compliance across multiple markets with a unified platform.

Facing these Challenges?

Frequent Regulatory Updates
Constant changes in global regulations require real-time monitoring and adjustments.
Coordination Among Multiple Stakeholders
RSMS simplifies compliance tracking across various regulatory bodies.

Risk of Non-Compliance
Mitigate risks of penalties, product recalls, and import alerts with proactive assessments.

Inefficient Manual Tracking
Eliminates manual errors by automating regulatory compliance processes.

Global Regulatory Coordination
Ensures seamless collaboration across different regulatory regions.
Features of AmpleLogic
Regulatory Surveillance Management System

Automated Regulatory Surveillance
Continuously monitors and analyzes regulatory changes from global agencies, providing real-time updates on evolving compliance requirements.

Impact Assessments for Compliance Risks
Automatically assesses the impact of new regulations on existing processes, helping organizations plan proactive mitigation strategies to reduce compliance risks.

Compliance Task Management
Assigns compliance tasks to the right teams with real-time tracking and notifications, ensuring accountability and preventing missed deadlines.

Regulatory Documentation Management
Integrates with Document Management Systems (DMS) to link regulatory changes directly to required documents, ensuring a centralized repository for compliance records and seamless audits.

Audit Trail & Reporting
Maintains a detailed record of all regulatory actions taken by the organization and generates compliance reports for internal reviews, audits, and regulatory submissions.

Cross-Border Compliance Management
Enables organizations to manage compliance across multiple regulatory jurisdictions, ensuring consistent adherence to varying regional compliance standards.

System Integration & Mobile Accessibility
Integrates seamlessly with QMS, ERP, LIMS, and other enterprise systems for smooth compliance management, while providing mobile access for real-time regulatory updates and compliance tracking.
Why Choose AmpleLogic's RSMS?
AmpleLogic’s RSMS is designed based on critical impact assessments, enabling pharmaceutical companies to track and mitigate compliance risks efficiently. Our system is tailored to address key compliance challenges, including:
- 483 Observations – Stay ahead of regulatory inspections and avoid observations by ensuring proactive compliance.
- Warning Letters – Identify patterns in regulatory warning letters and implement corrective actions before issues escalate.
- Import Alerts – Track and manage regulatory import alerts that may affect product distribution across global markets.


