AI-Powered APQR Software
Annual Product Quality Reviews, Reimagined with AI
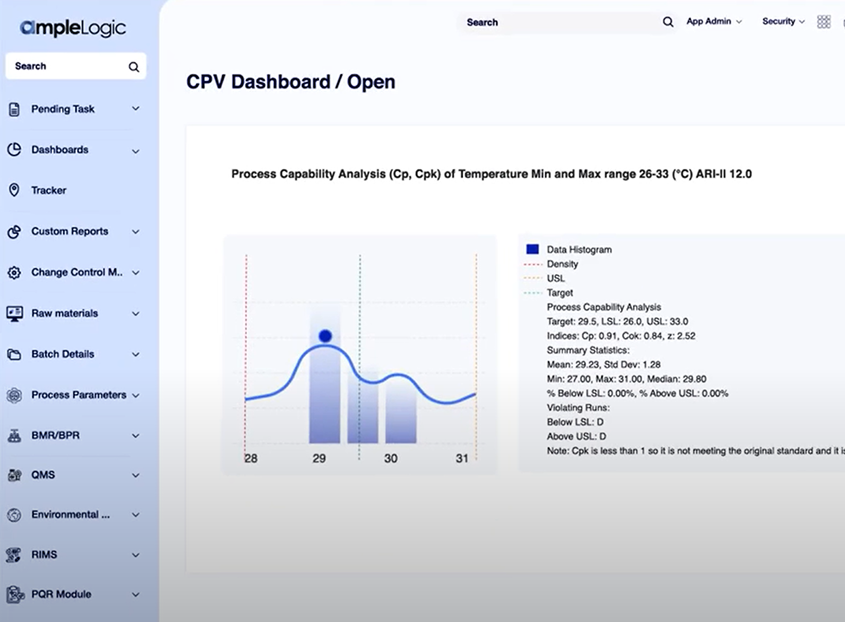
Annual Product Quality Review is a critical regulatory requirement for pharmaceutical and life sciences organizations. AmpleLogic’s APQR solution enables structured, compliant, and inspection-ready reviews by consolidating quality data, automating trend analysis, and generating audit-ready reports.
Trusted by 100+ Pharma Companies Globally






















Transforming Access Management in Life Sciences
AmpleLogic AI User Access Management (UAM) Software enhances security and compliance within the life sciences industry. AI-driven user provisioning automation ensures seamless and timely access management across applications like ERP, LIMS, and QMS. The software uses predictive analytics to identify potential access risks, providing proactive measures to prevent unauthorized access. AI also streamlines audit readiness by continuously monitoring user activities, detecting anomalies, and ensuring compliance with FDA and GMP standards. Additionally, AI-driven insights help optimize user access workflows, improving efficiency and reducing human error in managing complex systems.

Efficient Access Provisioning
Automated onboarding and offboarding processes streamline user life cycle management

Seamless Integration
Integration with Active Directory, SAP, HRMS, and LDAP ensures a cohesive user access system

Regulatory Compliance
Adherence to FDA, MHRA, ISO, EU Annex 11 and other global standards

Optimized License Management
A sophisticated license manager ensures efficient utilization and prevents unnecessary costs
User Access Management Challenges in Pharma

Regulatory Compliance
Ensuring compliance with strict regulations like FDA 21 CFR Part 11, GMP, and GxP is a significant challenge. UAM systems must enforce controls to prevent unauthorized access to sensitive data and ensure that user activities are properly logged for audits
Data Security
Protecting confidential and proprietary information, such as research data, manufacturing processes, and patient records, requires robust UAM systems. Unauthorized access or data breaches can lead to significant legal, financial, and reputational damage

Complex User Roles
Pharmaceutical organizations often have a wide range of users with varying roles and responsibilities. Managing access based on job functions, responsibilities, and regulatory requirements can be complex, particularly when users have access to multiple systems (ERP, LIMS, QMS, etc.)

Audit Readiness
Maintaining an auditable trail of user activity for regulatory inspections and compliance reviews is crucial. Ensuring that audit logs are comprehensive, accurate, and easily accessible can be difficult without an automated system

Integration with Multiple Systems
Integrating UAM across diverse legacy and modern pharmaceutical systems (ERP, HRMS, SAP) poses a significant challenge, balancing security, compliance, and seamless functionality.

Scalability
As pharmaceutical companies grow or expand globally, UAM systems must scale to handle a growing user base and comply with varying regional regulations. Ensuring consistency in user access policies across regions is critical for global compliance
Case Studies
Unleashing the Power of
AmpleLogic AI UMS

Audit Trail Automation
AI generates detailed, automated audit trails to ensure transparency and facilitate quick, efficient audits.

Application and License Manager
Effortlessly manage user licenses and permissions across software applications.

User Life Cycle Management
Automates onboarding and offboarding processes while seamlessly populating Active Directory.

User Access Reports
Systematically records user access data to generate reports on active and deactivated users and track employment details.

AD Account Creation & Deactivation
Bulk Active Directory accounts can be created instantly, while streamlined deactivation helps balance software and instrument licenses.

Unique Username
Generates a unique username for each employee that cannot be reused by another user.

Role Addition & Deletion
Manage role additions and deletions across software applications by viewing currently assigned roles.

Electronic Access Requests
Web-based system for efficient, paperless access request management.

Secure Audit Trails
Generates comprehensive audit reports with detailed user duties and e-signatures.

Changes in Role Permissions
Configure permission levels based on organizational structure and compliance needs.

User Access Permission Reports
Automatically generates reports showing software and instrument access for users.

Account Deactivation Instantly
Sends email notifications for scheduled deactivations, supporting effective license management.

Managing Compliance Over Time
Adheres to regulatory standards including FDA 21 CFR Part 11, MHRA, ISO, and EU Annex 11.

Enhanced Efficiency
Responds quickly to user needs, ensuring improved service delivery and operational efficiency.

Cost Reduction
Improves cost efficiency and employee productivity while meeting legal and regulatory requirements.

Detailed User Role and Duty Reporting
Custom reports outlining specific user duties within each software or instrument to enhance visibility and compliance.

Plant-Level Access and Permissions Reports
Plant-specific reports showing active and inactive users with associated access across multiple locations.

Instant Email Notifications for Account Deactivation
Sends instant alerts to administrators when user account deactivation is scheduled.

Centralized Licence Utilization Insights
Unified view of active, inactive, and duplicate licenses across systems for optimal license management.

Automated User Lifecycle Management
Streamlines onboarding and offboarding with fast, accurate access provisioning.

Effortless Integration
Integrates seamlessly with Active Directory, SAP HRMS, and LDAP for centralized access control.
Industries We Serve
Industry-Specific Software for Enhanced Quality and Compliance

Life Sciences
Benefit from GMP solutions customized for life sciences, Pharma, R&D, biotech etc.

Pharmaceuticals
Ensure consistent and inspection-ready APQR execution aligned with regulatory requirements.

Medical Devices
Transform the medical device industry with software that enhances compliance.

Gene & Cell Therapy
Address the complexity of advanced therapies by capturing batch-specific data, deviations.

Food & Beverages
Enhance food and beverage manufacturing with software for efficiency and operational agility.

Cosmetics
Maintain standardized APQR documentation and quality trend visibility to support regulatory.
Case Studies
Why choose AmpleLogic UMS?

Automated Access Requests
Eliminate manual paperwork, ensuring quicker and error-free access requests.

Comprehensive Audit Trails
Generate detailed audit reports with e-signatures for compliance and security assurance.

Role-Based Permissions
Effortlessly manage permission levels based on organizational structures. With AmpleLogic UMS, experience unparalleled efficiency and security.

Security
Ensures protection and integrity of data stored electronically, safeguarding it from unauthorized access, alteration, or loss.
Our compliance
Why Choose us?
Our compliance-first, AI-enabled platform is designed to meet the rigorous demands of regulated industries, enabling faster application.

10X Faster Deployment
Our platform accelerates solutions, reaching markets 10x faster.

ROI in 3 Months
Low-code solutions deliver fast deployment, cost savings, & quick ROI.

98% Project Rate
Experience a 98% success rate, surpassing the industry average of 56%

Domain Expertise
We deliver tailored solutions, ensuring business growth and lasting success
FAQ’s
Get Answers to All Your Queries
The system automates the process, seamlessly integrating with HR systems and Active Directory for efficient onboarding.
Absolutely, the system efficiently manages user licenses and permissions across a wide array of software applications.
AmpleLogic UAM adheres to US FDA, MHRA, ISO, EU and other global regulatory bodies providing a secure and compliant environment.
The system ensures prompt deactivation of accounts, preventing security vulnerabilities and optimizing license usage.
Yes, it’s adaptable to various industries including biologics, medical devices, chemical, manufacturing, food & beverages, cosmetics and more.





