AmpleLogic LMS
Best AI-Powered Learning Management System
Empower Your Workforce with our Revolutionary AI Solution
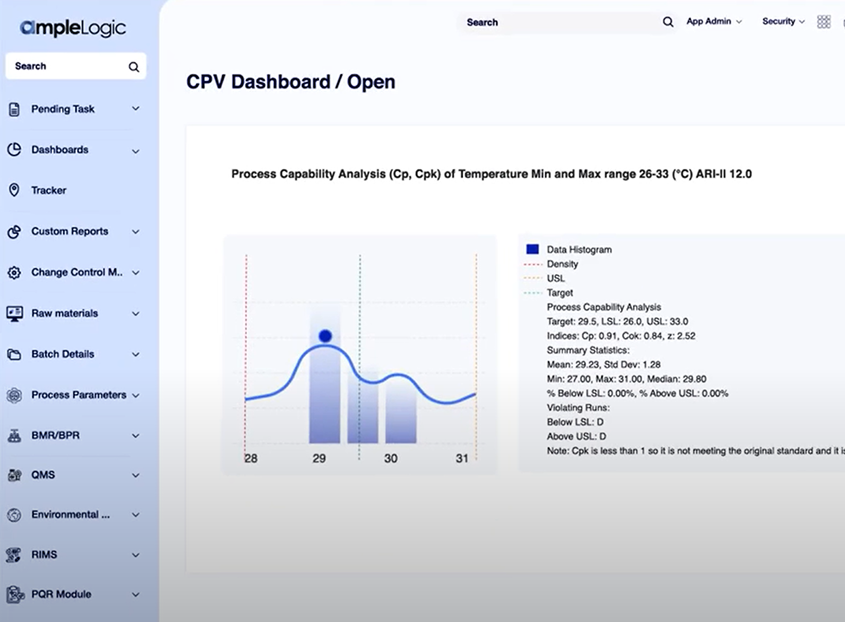
AI-Powered APQR Software
Annual Product Quality Reviews, Reimagined with AI
Annual Product Quality Review is a critical regulatory requirement for pharmaceutical and life sciences organizations. AmpleLogic’s APQR solution enables structured, compliant, and inspection-ready reviews by consolidating quality data, automating trend analysis, and generating audit-ready reports.
Trusted by 100+ Pharma Companies Globally






















Comprehensive Training Solution Powered with AI!
When it comes to workforce training and evaluation, pharmaceutical companies depend on effective electronic learning management system to streamline processes and enhance learning outcomes. One challenge, however, plagues the market today and that is personalized training of employees. Training and evaluation of personnels have been very generic overall that hinders the growth and overlooks the potential of individuals. This is where AmpleLogic AI-enabled LMS makes a difference. The solution allows organizations design tailored evaluation for each employee, leveraging AI-driven prompts that save time and enhances precision.
The AI-based SaaS solution is specifically crafted for regulated industries such as Lifesciences, Food & Beverages, Cosmetics, Gene Therapy, and Medical Devices. It simplifies the management of diverse training needs, including Standard Operating Procedures (SOPs), Current Good Manufacturing Practices (CGMP), and Compliance Training, ensuring employees are well-prepared to meet industry standards.
Commitment to AI-Powered Excellence
Innovative
Supports various learning resource formats that make learning fun

Global Accessibility
Access training materials anytime, anywhere, fostering flexibility

Regulatory Compliance
Fulfil FDA 21 CFR Part 11 and other regulatory standards effortlessly
AI-based Questionnaire Generation
Intelligent AI-Driven Questionnaire Generation

Facing these Challenges?

Compliance and Regulatory Requirements
Ensuring training programs meet strict standards like GMP and SOP is critical. Non-compliance can result in fines or loss of certification, and real-time tracking is essential for adherence
Data Security, Privacy, and Integration
LMS platforms must comply with data privacy laws (e.g., GDPR, HIPAA) and integrate with existing systems (ERP, CRM) for seamless data flow and compliance tracking

Inconsistent Training Delivery
Variations in training across regions can lead to knowledge gaps, affecting productivity and adherence to best practices. Uniform training is necessary to maintain high standards.

User Engagement and Scalability
Engaging employees in complex training is challenging, and as companies grow, the LMS must scale effectively to accommodate a larger workforce

Customization and Cost
Tailored training programs are needed for different departments, but implementing and maintaining an LMS can be costly, especially for smaller organizations

Cultural and Language Barriers
Global companies must ensure training content is culturally appropriate and available in multiple languages to meet diverse workforce needs
Features of AmpleLogic AI-Integrated LMS Software

Course Creation and Management
Centralize training resources, integrate with Document Management Software for easy SOP access, and create SCORM/xAPI-compliant courses with multimedia for enhanced learning.

User Management
Generate certificates after course completion, set expiration dates, and manage timely renewal alerts.

Progress Tracking
Real-time dashboards, detailed analytics, completion certificates, and timely performance insights.

Interactive Learning
Virtual classrooms, discussion forums, and Q&A sections for engaging and collaborative learning experiences.

AI-Based Questionnaire Generation
Automates question bank creation to streamline evaluations and reduce manual effort.

Customizable Learning Paths
Create personalized learning journeys based on job roles, departments, or career stages, ensuring role-based training alignment.

Skill Matrix
Gain insights into employee skill levels, identify gaps, and track improvements through comprehensive reporting.

Induction Training and Employee Management
Customize orientation programs, automate job-specific training for new hires, and track role updates.

Induction and cGMP Training at the Time of Joining
Tailored induction and cGMP training for new and transferred employees based on experience and required competencies.

On-the-Job Training
Plan, assess, and validate on-the-job training with structured competency evaluations.

Certifications & Alerts
Issue certifications, define validity periods, and trigger renewal alerts automatically.

Security and Data Privacy
Robust security controls to protect sensitive data, compliant with GDPR, HIPAA, and data privacy regulations.

Multi-language Support
Supports multiple languages, enabling accessibility for a diverse global workforce.

User Enrolment or Self-Registration
Create, manage, and allocate users to courses by department, with self-registration for role-specific training.

Cost Efficiency
Reduce training costs with virtual and self-paced learning while maintaining effectiveness.

Test Scores, Grades, and Results
Track test scores, grades, and overall learner performance with actionable insights.

Easy Integration
Seamlessly integrates with HR, CRM, and enterprise systems for a unified training ecosystem.

Training Sessions Schedule
Plan, reschedule, and track training sessions and attendance with qualified trainers.

Email Alerts, Notifications, and Escalation
Automated reminders, alerts, and escalations for upcoming training, overdue courses, and events.

Role-Based Training
Assign training based on job roles, automate validations, and send reminder notifications.

AI-based Assignment & Test
Create AI-driven assignments and exams, auto-assign courses, and generate complex assessments.

In-Depth Analytics & Reporting Tools
Advanced analytics to track learner progress, identify gaps, and enhance training effectiveness.

Bio-Metrics System Integration
Biometric fingerprint integration for secure and accurate attendance tracking.

Instructor-Led Training (ILT)
Organize and track instructor-led training sessions with biometric attendance support.

Robust User Engagement Tracking
Detailed engagement reports to identify support needs and continuously improve training effectiveness.
Switch to AmpleLogic AI-Based Pharma LMS Software
AmpleLogic’s AI-powered Pharmaceutical Management Training Software delivers centralized training management for regulated industries. Features include customized programs, on-the-job training, AI-driven questionnaires, and third-party integration. Fully compliant with 21 CFR Part 11, EU Annex 11, ISO, MHRA, GMP, and cGMP, it boosts compliance, productivity, and accessibility. This role based learning management software ensures each employee receives training suited to their job responsibilities.
Industries We Serve
Industry-Specific Software for Enhanced Quality and Compliance

Life Sciences
Apply Nelson Rules to detect abnormal process behaviour and statistically significant trends.

Pharmaceuticals
Ensure consistent and inspection-ready APQR execution aligned with regulatory requirements.

Medical Devices
Transform the medical device industry with software that enhances compliance.

Gene & Cell Therapy
Address the complexity of advanced therapies by capturing batch-specific data, deviations.

Food & Beverages
Conduct structured product quality reviews that support safety, consistency, and regulatory expectations.

Cosmetics
Maintain standardized APQR documentation and quality trend visibility to support regulatory.
Why choose AmpleLogic LMS Compliance Training Software?
Empower your team with AmpleLogic’s AI integrated LMS solution, offering comprehensive training solutions for regulated industries. Enjoy centralized course management, customized induction programs, on-the-job training planning, seamless integration with third-party systems and AI-enabled questionnaire generation. Elevate compliance, productivity, and accessibility with a globally compliant system that ensures compliance with regulatory frameworks such as 21 CFR Part 11, EU Annex 11, ISO, MHRA, GMP, and cGMP standards, providing a robust compliance foundation. The electronic learning management system ensures smooth access to training and tracking, while the role based learning management software aligns learning to employee roles.

Quick Notifications
Get immediate notifications for trainings, overdue courses and important events

On-the-Job Training
Plan and assess on-the-job training, ensuring competence in specific job functions with competency evaluation.

Personalized Training
Trainings are conducted in accordance with job roles

Biometric Attendance
Track attendance securely using biometric fingerprint technology
Our compliance
Why Choose us?
Our compliance-first, AI-enabled platform is designed to meet the rigorous demands of regulated industries, enabling faster application.

10X Faster Deployment
Our platform accelerates solutions, reaching markets 10x faster.

ROI in 3 Months
Low-code solutions deliver fast deployment, cost savings, & quick ROI.

98% Project Rate
Experience a 98% success rate, surpassing the industry average of 56%

Domain Expertise
We deliver tailored solutions, ensuring business growth and lasting success
FAQ’s
Get Answers to All Your Queries
AmpleLogic LMS adheres to compliances using audit trails, electronic signatures, and password authentication.
Yes, AmpleLogic LMS offers global accessibility, allowing users to access the platform anytime, anywhere.
The LMS generates detailed reports, showing employee roles, training history, and overall performance.
Yes, departments can plan and assess on-the-job training with AmpleLogic LMS, ensuring competence in specific job functions.
Yes, the system seamlessly integrates with quality management systems, document management systems and other existing applications.
Yes, we do. At AmpleLogic, we specialize in providing a robust Learning Management System (LMS) tailored for the food manufacturing industry. Our LMS is designed to streamline training processes, ensure compliance with food safety standards, and enhance operational efficiency.


