AI-Powered APQR Software
Annual Product Quality Reviews, Reimagined with AI
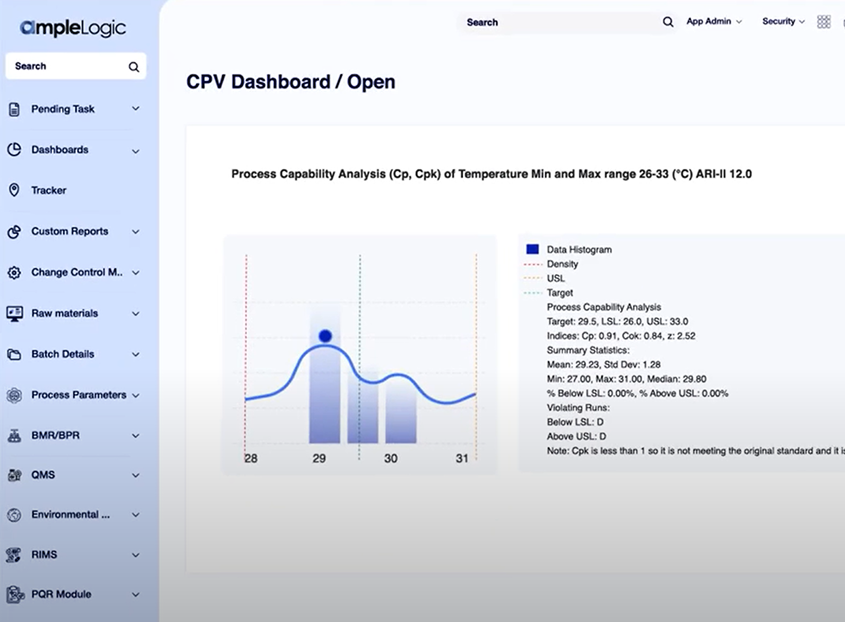
Annual Product Quality Review is a critical regulatory requirement for pharmaceutical and life sciences organizations. AmpleLogic’s APQR solution enables structured, compliant, and inspection-ready reviews by consolidating quality data, automating trend analysis, and generating audit-ready reports.
AI-Powered APQR Software
Annual Product Quality Reviews, Reimagined with AI
Annual Product Quality Review is a critical regulatory requirement for pharmaceutical and life sciences organizations. AmpleLogic’s APQR solution enables structured, compliant, and inspection-ready reviews by consolidating quality data, automating trend analysis, and generating audit-ready reports.
Trusted by 100+ Pharma Companies Globally






















Built to Support Modern Regulatory Expectations
Centralized APQR Data Collection
Consolidate inputs from deviations, CAPA, complaints, OOS/OOT, stability studies, and manufacturing data into a single, controlled APQR workspace.
Legible, Compliant Records
Easy Access for Cross-Functional Teams
Secure Audit Access
Always Inspection-Ready with AmpleLogic APQR
Regulatory inspections demand accurate, complete, and traceable product quality data often with very little notice. Manual APQR processes and disconnected systems make it difficult to retrieve consistent information, respond confidently to auditor questions, and demonstrate control over quality trends.
In regulated environments, inspection readiness is not a one-time activity—it must be continuously maintained. APQR data is often requested across multiple years, products, and sites, requiring organizations to demonstrate not only outcomes but also the decision-making process behind them. Without a centralized system, this becomes reactive, time-consuming, and error-prone. AmpleLogic APQR ensures that every review cycle is documented, approved, and preserved in a controlled manner.
All inputs, analyses, conclusions, and corrective actions are linked and traceable, providing a clear audit trail that supports regulatory expectations for transparency and accountability. By standardizing APQR execution and embedding compliance controls into daily operations, organizations can move away from last-minute data gathering and manual reconciliation. Instead, quality and regulatory teams gain continuous visibility into product performance, enabling faster responses to inspection queries and greater confidence in audit outcomes.
Enhancing APQR with Intelligent Automation
Annual Product Quality Reviews involve large volumes of structured and unstructured data from multiple systems and departments.
AI-Assisted Data Analysis
Automatically identify trends, anomalies, and recurring quality issues across review cycles.

OCR-Based Data Capture
Extract relevant data from scanned documents and legacy records to reduce manual effort.

AI-Generated Summaries
Create intelligent summaries of APQR findings to support faster decision-making and approvals.

Key Functional Capabilities

Six-Pack APQR Report Generation
Automatically generate standardized Six-Pack APQR reports by consolidating quality.

Product-wise Trend Analysis
Analyze quality trends across individual batches and entire product portfolios.

Master Data Management Products
Maintain controlled master data for products, manufacturing sites, batches, and quality parameters.

Nelson Rule-Based Statistical Analysis
Apply Nelson Rules to detect abnormal process behaviour and statistically significant trends.

Golden Batch Identification
Identify optimal manufacturing conditions by analyzing historical batch data, helping teams.

Automatic Version Control
Track every change to APQR records, analyses, and reports with complete version control.
Built-In Insights Without External Tools
AmpleLogic APQR includes integrated analytics and dashboards to visualize quality trends, risks, and performance indicators eliminating dependency on external reporting tools.
APQR Designed for Regulated Manufacturing
AmpleLogic’s APQR solution is purpose-built to support regulated manufacturing environments where product quality, data integrity, and inspection readiness are critical.

Life Sciences
Apply Nelson Rules to detect abnormal process behaviour and statistically significant trends.
Pharmaceuticals
Ensure consistent and inspection-ready APQR execution aligned with regulatory requirements.

Medical Devices
Manage APQRs with controlled documentation, version history, and traceability to support quality system.

Gene & Cell Therapy
Address the complexity of advanced therapies by capturing batch-specific data, deviations.

Food & Beverages
Conduct structured product quality reviews that support safety, consistency, and regulatory expectations.

Cosmetics
Maintain standardized APQR documentation and quality trend visibility to support regulatory.
Case Studies
Why Choose AmpleLogic APQR
AmpleLogic APQR is designed to help regulated organizations conduct Annual Product Quality Reviews with greater confidence, consistency, and efficiency. Built specifically for compliance-driven environments, the solution combines structured workflows, intelligent automation, and scalable architecture to support inspection-ready APQR execution across products and sites.

Purpose-Built for Regulated Environments
Designed to meet regulatory expectations for data integrity, traceability, and audit readiness, AmpleLogic APQR embeds compliance controls.

AI-Enabled Insights for Proactive Quality Management
Leverage intelligent automation to identify trends, anomalies, and recurring quality issues early enabling teams to move from reactive reporting.

Reduced APQR Cycle Time and Manual Effort
Automated data consolidation, analysis, and reporting significantly reduce manual effort, allowing teams to complete APQRs faster.

Scalable Across Products, Sites, and Geographies
Manage APQRs consistently across multiple products, manufacturing sites, and regions through a centralized platform designed to scale.
Our compliance
Why Choose us?
Our compliance-first, AI-enabled platform is designed to meet the rigorous demands of regulated industries, enabling faster application.

10X Faster Deployment
Our platform accelerates solutions, reaching markets 10x faster.

ROI in 3 Months
Low-code solutions deliver fast deployment, cost savings, & quick ROI.

98% Project Rate
Experience a 98% success rate, surpassing the industry average of 56%

Domain Expertise
We deliver tailored solutions, ensuring business growth and lasting success
Challenges with Traditional APQR
Fragmented Data Across Multiple Systems

Manual Consolidation & Spreadsheet Dependency

Inconsistent Formats & Review Practices

Limited Visibility into Quality Trends

Increased Inspection & Compliance
FAQ’s
Get Answers to All Your Queries
GAMP stands for Good Automated Manufacturing Practice. It’s a set of guidelines and best practices for the pharmaceutical industry to ensure that automated systems are properly designed, validated, and maintained.
AmpleLogic GAMP Solutions provide compliant digital solutions that help organizations meet regulatory requirements through validated systems and documentation.
Compliance is ensured through validation frameworks, audit trails, documentation, and adherence to global regulatory guidelines.
aPaaS (Application Platform as a Service) enables rapid application development, deployment, and management in a compliant environment.
Key features include compliance-ready workflows, audit trails, validation support, scalability, and enterprise security.
Data integrity is ensured through access controls, encryption, audit logs, and compliance with ALCOA+ principles.





